Measurement traceability for 3D printing
Challenge
More than 500 000 medical devices are on sale in Europe, generating around €51 billion annually and accounting for one third of the market worldwide. This sector could potentially benefit from the rapidly expanding additive manufacturing (AM) industry. Commonly known as 3D printing this technique could allow the low-cost fabrication of parts for such things as implants for facial reconstruction, knee joint replacements or grafts that promote bone growth, that are specifically tailored to a patient’s needs. However, the layer-wise manufacturing method this process uses, combined with the often-high roughness, complex geometries, materials and internal structures of AM products, makes acquiring accurate data challenging. This can lead to final parts that do not meet the initial patient-specific specifications, which had restricted its uptake by manufacturers of medical implants as they required SI traceable techniques for quality control to verify the finished product. Validated measurements were therefore needed covering the entire manufacturing chain - from the initial medical scans to the final implantation of the printed part into the patient.
Solution
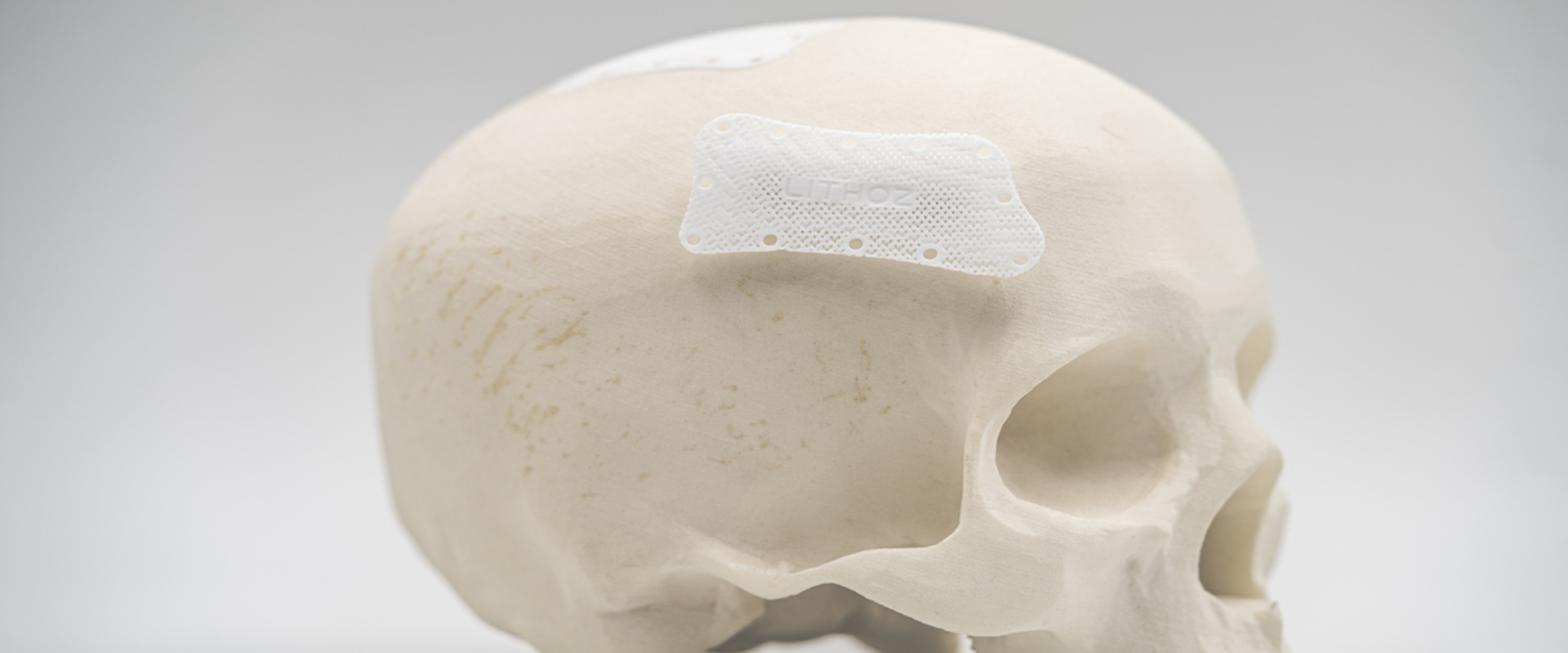
The EMPIR project Metrology for additively manufactured medical implants brought together experts from national metrology institutes (NMI), industry and academia to address the need for AM measurement traceability. More than 100 medical implants, guides and standard objects were fabricated using different 3D printing technologies with a range of internal structures composed of materials such as polymers, ceramics, and metals. These were characterised for density, porosity, surface roughness and defects using over 200 destructive and non-destructive measurement techniques. A detailed analysis was performed using X-ray or terahertz computed tomography (CT), which provide a volumetric (external and internal geometries) three-dimensional image of an object, and coordinate measurement machines, that measure the external geometry of objects using tactile probes. The quantification of manufacturing errors was examined for pedicle screw drills used in spinal surgery, maxillo-facial implants for cheekbone reconstruction, dental guides and cranial implants. This resulted in the first ever knowledge of the build-up of measurement uncertainties across the whole AM chain; from the scan of the patient to eventual clinical use, with full traceability to the SI.
Impact
Since its founding in 2011, the company Lithoz has become the world market and technology leader for 3D printers, materials and solutions for the industrial production of high-performance ceramics. As a project partner the company was involved with the fabrication of AM parts. They are now passing on the knowledge gained - especially for non-destructive computed tomography measurements – to their customers who are now using this information to feed into their production environments. Furthermore, overcoming the measurement challenges that Lithoz faced during the project has played a part in stimulating the development of their new database driven CeraFab Control software. This allows complete traceability, quality assurance and intelligent data analysis to give greater efficiency in AM for ceramic parts. As a result of the more stable and robust processes developed in the project Lithoz has also seen an increase in customer confidence in the use of AM for production. For the first time measurement traceability is available for 3D printed medical implants. This will enable the medical technology sector to benefit from this innovative manufacturing technique, an important step towards increased access to high-quality, low-cost medical devices across Europe.
- Category
- EMPIR,
- Health,