Innovation in molecular radiotherapy
Challenge
In 2020, 2.7 million people in the European Union were diagnosed with cancer and 1.3 million lives were lost to the disease. The EU Commission supports research to improve prevention, detection, diagnosis and treatment of cancer. Quality of life for patients during treatment is also a priority, and is an important factor for evaluating new therapies. Molecular radiotherapy (MRT) has been shown to slow the progression of inoperable liver cancers and offer reduced risks of adverse effects compared to existing therapies. Selective Internal Radiation Therapy (SIRT), a type of MRT, involves injecting tiny radioactive ‘microspheres’ that selectively lodge in or near tumours to deliver intense and targeted doses while sparing healthy tissue.
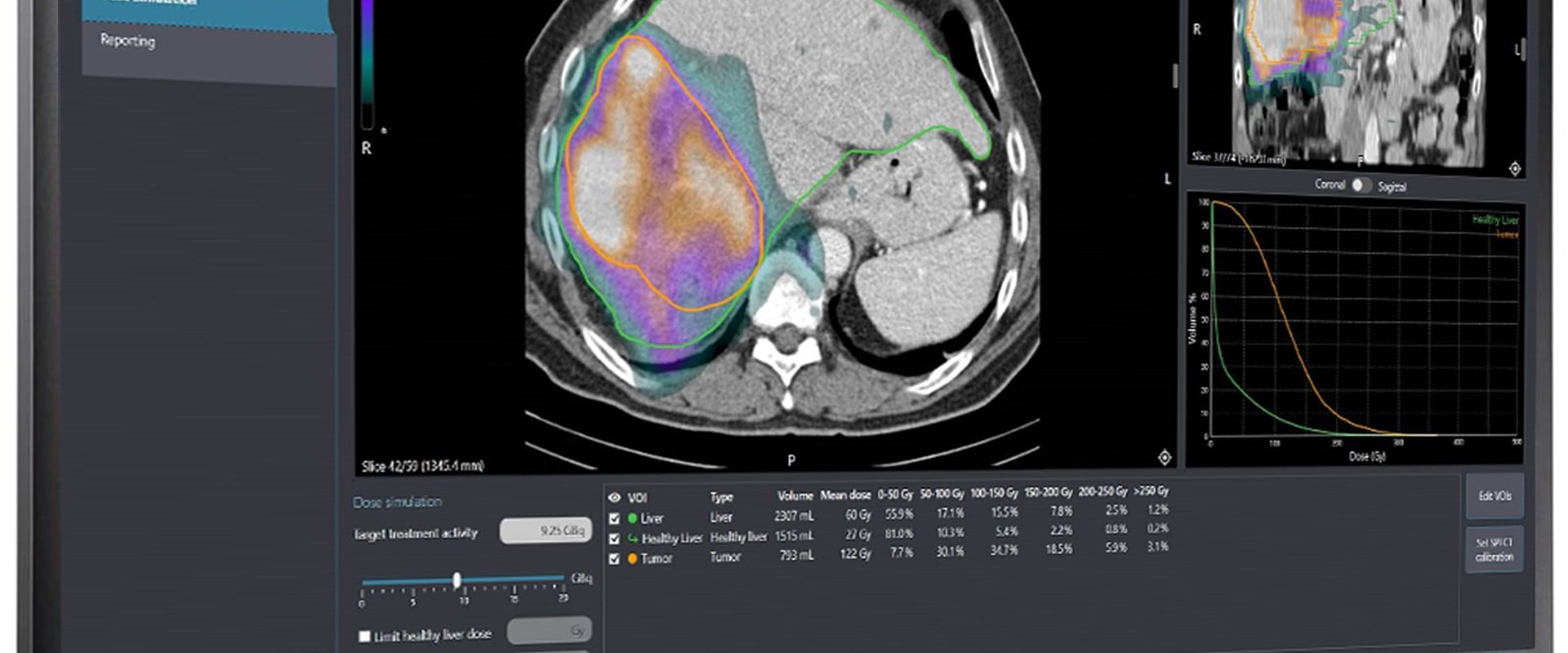
Holmium-166 (166Ho) is a high-energy beta-emitting isotope that offers advantages in SIRT due to some unique properties. A 26.8-hour half-life means each dose is effectively delivered within four days of injection. Paramagnetic properties enable microsphere, and therefore cancer cell, locations to be tracked inside patients using magnetic resonance imaging, even well after administration. A particular advantage compared to other isotopes results from 166Ho also being a gamma-radiation emitter, allowing Single Photon Emission Computed Tomography (SPECT) to also be used to both visualise microsphere location and directly measure absorbed dose, so enabling personalised dosimetry.
Studies showed SIRT treatment response is directly related to absorbed dose, the energy absorbed by tumour cells. However, without practical procedures to calibrate SPECT imaging equipment and radionuclide calibrators, clinics could only deliver nominal doses insufficiently adjusted to each patient. Lack of specific calibration procedures also made it harder for radiopharmaceutical companies to innovate, while EC Directive 2013/59/EURATOM, requiring clinics to provide individualised doses to radiotherapy patients, made MRT adoption problematic.
Solution
The EMPIR project 'Metrology for clinical implementation of dosimetry in molecular radiotherapy' developed calibration protocols as well as guidance on commissioning and quality control for quantifying SPECT imaging and the calculation of absorbed dose. These protocols built on those developed in the EMRP MetroMRT project, expanded to enable accurate calibrations of SPECT imaging for a wider range of radionuclides. This enabled dose calibration and quantitative imaging to be performed in clinical settings, traceable to primary radioactivity standards.
Impact
Quirem Medical, a University Medical Center Utrecht spin-off company latterly a subsidiary of Terumo Europe, develops and commercialises radioactive microspheres based on 166Ho. It offers a delivery platform for administering ‘scout’ and treatment spheres, and its Q-Suite imaging software to guide physicians on effective delivery of personalised SIRT treatments.
The company discovered that users found it hard to tell whether complete doses were delivered from semi-transparent HDPE vials inside the platform. Aware of the expanded calibration procedures devised in the project, it engaged National Physical Laboratory (NPL), the UK National Metrology Institute, to calculate a calibration standard for the radionuclide and evaluate a calibration procedure for a revised platform that used clear-glass vials.
The resulting 166Ho calibration standard enabled Quirem Medical to provide more reliable patient dose planning and verification, as the radiopharmaceutical manufacturer could precisely calibrate doses for clinics, and clinics calibrate doses before each treatment. The collaboration also confirmed the suitability of the more user-friendly packaging design, which, according to the company, helped drive commercial adoption.
The project supported effective, better-targeted treatments, and helped clinics maintain compliance with regulations, so advancing the prospect of improved outcomes for cancer patients across Europe.
- Category
- Health,
- EMPIR,